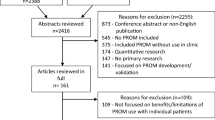
Abstract
A systematic review was conducted to identify best practices for increasing linkage, retention and re-engagement in HIV care (LRC) for persons living with HIV (PLWH). Our search strategy consisted of automated searches of electronic databases and hand searches of journals, reference lists and listservs. We developed two sets of criteria: evidence-based to identify evidence-based interventions (EBIs) tested with a comparison group and evidence-informed to identify evidence-informed interventions (EIs) tested with a one-group design. Eligible interventions included being published between 1996 and 2014, U.S.-based studies with a comparison or one-group designs with pre-post data, international randomized controlled trials, and having objective measures of LRC-relevant outcomes. We identified 10 best practices: 5 EBIs and 5 EIs. None focused on re-engagement. Providers and prevention planners can use the review findings to identify best practices suitable for their clinics, agencies, or communities to increase engagement in care for PLWH, ultimately leading to viral suppression.
Resumen
Una revisión sistemática se realizó para identificar las mejores prácticas para aumentar la vinculación, la permanencia y el regreso hasta atención médica del VIH (VPR) para las personas que viven con el VIH (PVVS). La estrategia de búsqueda consistió en búsquedas automatizadas de bases de datos electrónicas y búsquedas manuales en revistas, listas de referencias y listas de correo electrónico. Hemos desarrollado dos juegos de criterios: “basadas en evidencias” para identificar las intervenciones basadas en la evidencia y probadas con un grupo de comparación (IBEs), y “informadas por evidencias” para identificar las intervenciones informadas por evidencias y probadas con un diseño empleando un solo grupo (IIEs). Intervenciones elegibles incluyeron siendo publicados entre 1996 y 2014, estudiados en los Estados Unidos con un grupo de comparación o uno grupo con datos pre-post, ensayos internacionales controlados aleatorios, y que tienen medidas objetivas de resultados VPR-relevantes. Se identificaron 10 mejores prácticas: 5 IBEs y 5 IIEs. Ninguno se centró en un regreso hasta atención médica. Los proveedores y los planificadores de prevención pueden utilizar los resultados de la revisión para identificar las mejores prácticas adecuadas para sus clínicas, agencias, o comunidades para aumentar la participación en la atención médica para las PVVS, en última instancia conduciendo a la supresión viral.


Similar content being viewed by others
Notes
Although initiating ART is dependent on a health care professional, this element is an important step in the care continuum and demonstrates additional evidence of engagement in care.
Given that viral load suppression is considered the ultimate goal in the continuum of care, interventions demonstrating evidence of improvements in viral load suppression may be more effective than studies that do not.
References
Office of National AIDS Policy. National HIV/AIDS strategy for the United States: updated to 2020, Washington, DC: office of National AIDS Policy. https://www.aids.gov/federal-resources/national-hiv-aids-strategy/nhas-update.pdf (2015). Accessed 30 July 2015.
Bradley H, Hall HI, Wolitski RJ, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV—United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113–7.
Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505.
Yehia BR, French B, Fleishman JA, et al. Retention in care is more strongly associated with viral suppression in HIV-infected patients with lower versus higher CD4 counts. J Acquir Immune Defic Syndr. 2014;65(3):333–9.
Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5(6):e11068.
Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376(9740):532–9.
Cohen SM, Hu X, Sweeney P, Johnson AS, Hall HI. HIV viral suppression among persons with varying levels of engagement in HIV medical care, 19 US jurisdictions. J Acquir Immune Defic Syndr. 2014;67(5):519–27.
Crawford TN. Poor retention in care one-year after viral suppression: a significant predictor of viral rebound. AIDS Care. 2014;26(11):1393–9.
Mugavero MJ, Westfall AO, Cole SR, et al. Beyond core indicators of retention in HIV care: missed clinic visits are independently associated with all-cause mortality. Clin Infect Dis. 2014;59(10):1471–9.
Mugavero MJ, Lin HY, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–56.
Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175(4):588–96.
Higa DH, Marks G, Crepaz N, Liau A, Lyles CM. Interventions to improve retention in HIV primary care: a systematic review of U.S. studies. Curr HIV/AIDS Rep. 2012;9(4):313–25.
Liau A, Crepaz N, Lyles CM, et al. Interventions to promote linkage to and utilization of HIV medical care among HIV-diagnosed persons: a qualitative systematic review, 1996–2011. AIDS Behav. 2013;17(6):1941–62.
Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ Publication No. 10(14)-EHC063-EF. Rockville, MD: agency for healthcare research and quality. www.effectivehealthcare.ahrq.gov (2014). Accessed 10 Feb 2015.
Higgins JPT, Altman DG, Sterne JAC, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: assessing the risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0. http://handbook.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm (2011). Accessed 10 Feb 2015.
The Guide to Community Preventive Services (The Community Guide). http://www.thecommunityguide.org/index.html (2015). Accessed 12 Feb 2015.
Grading of Recommendations, Assessment, Development and Evaluation (GRADE). http://www.gradeworkinggroup.org/index.htm (2015). Accessed 10 Feb 2015.
Muhamadi L, Tumwesigye NM, Kadobera D, et al. A single-blind randomized controlled trial to evaluate the effect of extended counseling on uptake of pre-antiretroviral care in Eastern Uganda. Trials. 2011;12:184.
Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19(4):423–31.
Gardner LI, Giordano TP, Marks G, et al. Enhanced personal contact with HIV patients improves retention in primary care: a randomized trial in 6 US HIV clinics. Clin Infect Dis. 2014;59(5):725–34.
Lucas GM, Chaudhry A, Hsu J, et al. Clinic-based treatment of opioid-dependent HIV-infected patients versus referral to an opioid treatment program: a randomized trial. Ann Intern Med. 2010;152(11):704–11.
Robbins GK, Lester W, Johnson KL, et al. Efficacy of a clinical decision-support system in an HIV practice: a randomized trial. Ann Intern Med. 2012;157(11):757–66.
Mugavero MJ. Improving engagement in HIV care: what can we do? Top HIV Med. 2008;16(5):156–61.
Davila JA, Miertschin N, Sansgiry S, Schwarzwald H, Henley C, Giordano TP. Centralization of HIV services in HIV-positive African-American and Hispanic youth improves retention in care. AIDS Care. 2013;25(2):202–6.
Enriquez M, Farnan R, Cheng AL, et al. Impact of a bilingual/bicultural care team on HIV-related health outcomes. J Assoc Nurses AIDS Care. 2008;19(4):295–301.
Gardner LI, Marks G, Craw JA, et al. A low-effort, clinic-wide intervention improves attendance for HIV primary care. Clin Infect Dis. 2012;55(8):1124–34.
Hightow-Weidman LB, Smith JC, Valera E, Matthews DD, Lyons P. Keeping them in “STYLE”: finding, linking, and retaining young HIV-positive black and Latino men who have sex with men in care. AIDS Patient Care STDS. 2011;25(1):37–45.
Wanyenze RK, Kamya MR, Fatch R, et al. Abbreviated HIV counselling and testing and enhanced referral to care in Uganda: a factorial randomised controlled trial. Lancet Glob Health. 2013;1(3):e137–45.
Andersen M, Tinsley J, Milfort D, et al. HIV health care access issues for women living with HIV, mental illness, and substance abuse. AIDS Patient Care STDS. 2005;19(7):449–59.
Bocour A, Renaud TC, Udeagu CC, Shepard CW. HIV partner services are associated with timely linkage to HIV medical care. AIDS. 2013;27(18):2961–3.
Henry SR, Goetz MB, Asch SM. The effect of automated telephone appointment reminders on HIV primary care no-shows by veterans. J Assoc Nurses AIDS Care. 2012;23(5):409–18.
Keitz SA, Box TL, Homan RK, Bartlett JA, Oddone EZ. Primary care for patients infected with human immunodeficiency virus: a randomized controlled trial. J Gen Intern Med. 2001;16(9):573–82.
Keller S, Jones J, Erbelding E. Choice of Rapid HIV testing and entrance into care in Baltimore City sexually transmitted infections clinics. AIDS Patient Care STDS. 2011;25(4):237–43.
Konkle-Parker DJ, Erlen JA, Dubbert PM, May W. Pilot testing of an HIV medication adherence intervention in a public clinic in the Deep South. J Am Acad Nurse Pract. 2012;24(8):488–98.
Kunutsor S, Walley J, Katabira E, et al. Improving clinic attendance and adherence to antiretroviral therapy through a treatment supporter intervention in Uganda: a randomized controlled trial. AIDS Behav. 2011;15(8):1795–802.
Naar-King S, Outlaw A, Green-Jones M, Wright K, Parsons JT. Motivational interviewing by peer outreach workers: a pilot randomized clinical trial to retain adolescents and young adults in HIV care. AIDS Care. 2009;21(7):868–73.
Wohl DA, Scheyett A, Golin CE, et al. Intensive case management before and after prison release is no more effective than comprehensive pre-release discharge planning in linking HIV-infected prisoners to care: a randomized trial. AIDS Behav. 2011;15(2):356–64.
Perron NJ, Dao MD, Kossovsky MP, et al. Reduction of missed appointments at an urban primary care clinic: a randomised controlled study. BMC Fam Pract. 2010;11:79.
Willis S, Castel AD, Ahmed T, Olejemeh C, Frison L, Kharfen M. Linkage, engagement, and viral suppression rates among HIV-infected persons receiving care at medical case management programs in Washington, DC. J Acquir Immune Defic Syndr. 2013;64(Suppl 1):S33–41.
Centers for Disease Control and Prevention. Routinely recommended HIV testing at an urban urgent-care clinic–Atlanta, Georgia, 2000. Morb Mortal Wkly Rep. 2001;50(25):538–41.
Schrantz SJ, Babcock CA, Theodosis C, et al. A targeted, conventional assay, emergency department HIV testing program integrated with existing clinical procedures. Ann Emerg Med. 2011;58(1 Suppl 1):S85–8.
Craw JA, Gardner LI, Marks G, et al. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic Syndr. 2008;47(5):597–606.
Shrestha RK, Gardner L, Marks G, et al. Estimating the cost of increasing retention in care for HIV-infected patients: results of the CDC/HRSA retention in care trial. J Acquir Immune Defic Syndr. 2014. doi:10.1097/QAI.0b013e3182a99b67.
de Bruin M, Viechtbauer W, Schaalma HP, Kok G, Abraham C, Hospers HJ. Standard care impact on effects of highly active antiretroviral therapy adherence interventions: a meta-analysis of randomized controlled trials. Arch Intern Med. 2010;170(3):240–50.
Valdiserri RO, Forsyth AD, Yakovchenko V, Koh HK. Measuring what matters: development of standard HIV core indicators across the U.S. Department of Health and Human Services. Public Health Rep. 2013;128(5):354–9.
de Bruin M, McCambridge J, Prins JM. Reducing the risk of bias in health behaviour change trials: improving trial design, reporting or bias assessment criteria? A review and case study. Psychol Health. 2015;30(1):8–34.
Mugavero MJ, Westfall AO, Zinski A, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;61(5):574–80.
Centers for Disease Control and Prevention. High impact HIV-prevention: CDC’s approach to reducing HIV infections in the United States. http://www.cdc.gov/hiv/pdf/policies_NHPC_Booklet.pdf (2015). Accessed 13 Mar 2015.
Centers for Disease Control and Prevention, Health Resources and Services Administration, National Institute of Health et al. Recommendations for HIV prevention with adults and adolescents with HIV in the United States, 2014. http://stacks.cdc.gov/view/cdc/26062 (2014).
Cunningham CO, Li X, Ramsey K, Sohler NL. A comparison of HIV health services utilization measures in a marginalized population: self-report versus medical records. Med Care. 2007;45(3):264–8.
Acknowledgments
The authors would like to acknowledge current and former members of the Prevention Research Synthesis Project (PRS) who helped with the review (alphabetical order: Adebukola Adegbite, Brittney Baack, Terrika Barham, Julia Deluca, Linda Kay, Cindy Lyles, Khiya Marshall, Theresa Sipe, Maria Louisa Tungol, H. Waverly Vosburgh, & Christina White) and the consultants who helped to develop the LRC best practices criteria (alphabetical order: Rivet Amico, Jason Craw, Arin Freeman, Lytt Gardner, Thomas Giordano, Cyndi Grossman, Janet Heitgerd, Lisa Hightow-Weidman, Linda Koenig, Gary Marks, Lisa Metsch, Michael Mugavero, David Purcell, Stephen Safren, Luke Shouse, Michael Stirratt, Raekiela Taylor, Amy Wohl).
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author information
Authors and Affiliations
Consortia
Corresponding author
Appendices
Appendix 1: CDC’s Prevention Research Synthesis Project Criteria for Evidence-Based Interventions (EBIs) for Linkage to, Retention in, and Re-engagement in HIV Care (LRC)
Quality–Study Design
-
Prospective or quasi-prospective study design
-
Appropriate and concurrent comparison arm, or appropriate non-concurrent comparison arm that was implemented in a different clinic or agency within 12 months of the start of the intervention and was similar in population and setting
-
Random allocation of participants to study arms or if non-randomization, potential bias in allocation to intervention is minimized
Quality–Study Implementation and Analysis
-
For linkage to care interventions, linkage to care occurred within or less than 6 months after the initiation of the intervention
-
For retention in care interventions, retention in care occurred at least 6 months after the initiation of the intervention
-
Comparison between intervention arm and an appropriate comparison arm
-
Analysis of participants in study arms as originally allocated, or contaminated participants may be excluded if numbers are small, but participants may not be re-assigned for analytic purposes
-
Analysis of participants may be based on intervention exposure, where participants exposed to <50 % of the entire intended intervention may be excluded
-
Analysis must be based on between-group comparisons on post-intervention levels or on pre-post changes in measures
-
For pre-post changes used in analysis, measures must be identical, including identical recall period
-
-
Analysis based on a 2-sided test with a p value <0.05
-
With nonrandomized assignment, either no statistical differences exist in baseline levels of the outcome measure, or baseline differences must be controlled for in the analysis. If moderately biased assignment or historical comparison was used, differences in baseline demographics also must be controlled for in the analysis
-
Baseline sample of ≥40 participants (or charts) per study arm
Strength of Evidence: Significant Positive Intervention Effects
-
Statistically significant (p < .05) positive intervention effect for ≥1 relevant outcome measure
-
A positive intervention effect is defined as an improvement in linking to, retention in, or re-engagement in HIV medical care in the intervention arm relative to the comparison arm
-
A relevant outcome is defined as an actual/completed outpatient primary HIV medical care visit or HIV viral load and/or CD4 count when used as proxies for a HIV medical care visit
-
Completed HIV medical visits must be documented in medical records, administrative or agency records, or surveillance reports
-
Self-reports of completed medical visits validated by medical records, administrative or agency records are also acceptable
-
-
-
For linkage to care, a relevant outcome is the actual/completed first HIV medical visit for newly diagnosed HIV-positive persons
-
For retention in care, a relevant outcome is having actual/completed multiple HIV medical visits over a period of time
-
For re-engagement in care, a relevant outcome is the actual/completed initial HIV medical visit for HIV-positive persons who have fallen out of, but have returned to, HIV care
-
Effect at a required follow-up assessment time point and based on the analyses that meets all study implementation and analysis criteria
Strength of Evidence: Significant Negative Intervention Effects
-
No statistically significant (p < .05) negative intervention effect for any relevant outcome
-
A negative intervention effect is defined as a worsening in linkage to, retention in, or re-engagement in HIV medical care in the intervention arm relative to the comparison arm
-
-
No other statistically significant harmful intervention effect that causes substantial concern
-
For an intervention with a replication evaluation, no significant negative intervention effects in the replication study if the intervention was implemented in the exact same way as the original study and with the same or similar cohort/population
Additional Limitations to Evaluate
-
No evidence that additional limitations resulted in considerable bias that reduces the confidence of the findings
-
Examples of limitations
-
Too many post hoc analyses
-
Inconsistent evidence between effects
-
Inappropriate subset analyses
-
Not accounting for various reasons why participants were not included in the LRC outcome
-
Not adjusting for cluster effects for studies that allocate individuals to a group-level intervention
-
Not accounting for factors that may influence findings, but are not attributable to the intervention (e.g., historical events)
-
Other notable biases threatening internal or external validity
-
-
All criteria must be satisfied for an intervention to be considered as a LRC Evidence-based Intervention (EBI).
Appendix 2: Evidence-Informed Interventions (EIs) for Linkage to, Retention in, and Re-engagement in HIV Care (LRC)
Quality–Study Design
-
Evaluates data before and after intervention implementation in studies without a comparison arm
Quality–Study Implementation and Analysis
-
For pre-post intervention changes, analysis based on a 2-sided test with a p value of <.05
Strength of Evidence: Significant Positive Intervention Effects
-
Statistically significant (p < .05) positive pre-post intervention effect for ≥1 relevant outcome measure
-
A positive intervention effect is defined as an improvement in linking to, retention in, or re-engagement in HIV medical care from pre to post intervention
-
A relevant outcome is defined as an actual/completed outpatient primary HIV medical care visit or HIV viral load and/or CD4 counts when used as proxies
-
For linkage to care, a relevant outcome is the actual/completed first HIV medical visit for newly-diagnosed HIV-positive persons
-
For retention in care, a relevant outcome is having actual/completed multiple HIV medical visits over a period of time
-
For re-engagement in care, a relevant outcome is the actual/completed initial HIV medical visit for HIV-positive persons who have fallen out of, but have returned to, HIV care
-
-
A positive intervention effect must be documented in medical records, administrative or agency records, or surveillance reports
-
Self-reports of medical visits validated by medical records, administrative or agency records are also acceptable
-
Strength of Evidence: Significant Negative Intervention Effects
-
No statistically significant (p < .05) negative pre-post intervention effect for any relevant outcome
-
A negative intervention effect is defined as a worsening in linkage to, retention in, or re-engagement in HIV medical care post intervention compared to the pre-intervention
-
-
No other statistically significant harmful intervention effect that causes substantial concern
Additional Limitations to Evaluate
-
No evidence that additional limitations resulted in considerable bias that reduces the confidence of the findings
-
Examples of limitations
-
Too many post hoc analyses
-
Inconsistent evidence between effects
-
Inappropriate subset analyses
-
Not accounting for various reasons why participants were not included in the LRC outcome
-
For serial cross-sectional studies, there are statistically significant differences in demographic characteristics between “pre” and “post” samples that may introduce bias
-
Other notable biases threatening internal or external validity
-
-
All criteria must be satisfied for an intervention to be considered as a LRC Evidence-informed intervention (EI).
Additional Study Strengths
All Evidence-informed studies that exhibit additional strengths will have those strengths noted on all summary documentation. These strengths include:
-
Study design-related strengths:
-
For studies using serial cross-sectional designs in a clinic setting, having comparable clinic samples across different times
-
-
Implementation-related strengths:
-
Outcomes occur within or exceed optimal follow-up assessment time points
-
Linkage or entry to care outcomes occur ≤3 months (follow up time point of at least 3 months)
-
Retention in care outcomes occur ≥12 months (follow up time point of at least 12 months)
-
Re-engagement outcomes that re-engage persons within 6 months of intervention initiation or retain persons re-engaged in care for 2 visits for at least 12 months after intervention initiation
-
Targeting persons who have been lost to care at least 12 months
-
Re-engagement studies that attempt to re-engage persons who have been lost to care 12 months or longer
-
-
Sample size
-
Linkage, retention, and re-engagement studies with sample sizes equal to or above 100
-
-
-
Impact-related strengths:
-
Post-intervention data or levels meet the National HIV/AIDS Strategy objectives
-
Percent of persons linked to HIV care post-intervention is at least 85 %
-
Percent of persons retained in HIV care post-intervention is at least 80 %
-
-
Study shows evidence of ART initiation from pre- to post-interventionFootnote 1
-
Linkage, retention, and re-engagement studies that demonstrate a statistically significant positive change or at least a 10 % increase in the percent of persons who initiate ART from pre- to post- intervention
-
-
The study shows evidence of improvements in viral load suppression from pre- to post- interventionFootnote 2
-
Linkage, retention, and re-engagement studies that demonstrate a statistically significant positive change or at least a 10 % increase in the percent of persons who are virally suppressed from pre- to post- intervention
-
-
Appendix 3: Search Strategy—MEDLINE OVID
HIV/HIV Positive Person MeSH and Keywords
-
(1)
HIV infections/co, dt, di, nu, pc, px, th, tm
-
(2)
HIV infect$.ti,ab
-
(3)
(HIV adj4 diagnos$).ti,ab
-
(4)
HIV positiv$.ti,ab
-
(5)
(HIV adj4 care).ti,ab
-
(6)
(HIV adj4 treatment$).ti,ab
-
(7)
living with HIV.ti,ab
-
(8)
or/1–7
Linking and Retention in Care Mesh and Keywords
-
(9)
(access$ adj4 care).ti,ab
-
(10)
(access$ adj4 barrier$).ti,ab
-
(11)
(access$ adj4 (treatment or service$)).ti,ab
-
(12)
(barrier$ adj4 care).ti,ab
-
(13)
case management.ti,ab
-
(14)
case manager$.ti,ab
-
(15)
(decreas$ adj4 barrier$).ti,ab
-
(16)
(engag$ adj4 (care or service$)).ti,ab
-
(17)
(enroll$ adj4 care).ti,ab
-
(18)
((enter$ or entry) adj4 care).ti,ab
-
(19)
((enter$ or entry) adj4 service$).ti,ab
-
(20)
(improv$ adj4 access$).ti,ab
-
(21)
(improv$ adj4 retention).ti,ab
-
(22)
((kept or keep$ or return$) adj4 appointment$).ti,ab
-
(23)
(link$ adj4 (retain$ or retent$)).ti,ab
-
(24)
(link$ adj4 care).ti,ab
-
(25)
(link$ adj4 case).ti,ab
-
(26)
(link$ adj4 treatment).ti,ab
-
(27)
(link$ adj4 service$).ti,ab
-
(28)
(outreach adj4 (care or link$ or program$)).ti,ab
-
(29)
((provision or provid$) adj4 (care or service$)).ti,ab
-
(30)
(reduc$ adj4 barrier$).ti,ab
-
(31)
((re engag$ or reengag$) adj4 (care or treatment or service$)).ti,ab
-
(32)
((re enter$ or reenter$) adj4 (care or treatment or service$)).ti,ab
-
(33)
((refer or refers or referred or referral$) adj4 (care or medical or treatment or clinic or service$)).ti,ab
-
(34)
((retain$ or retent$) adj4 care).ti,ab
-
(35)
(seek$ adj4 (care or treatment$)).ti,ab
-
(36)
(utiliz$ adj4 (treatment or care or service$)).ti,ab
-
(37)
(medical adj4 (care or treatment or service$)).ti,ab
-
(38)
(gap$ adj2 care).ti,ab
-
(39)
(visit adj2 (constan$ or consist$)).ti,ab
-
(40)
(appointment$ adj2 adher$).ti,ab
-
(41)
((follow-up or follow up) adj2 discontin$).ti,ab
-
(42)
((miss$ or schedul$) adj2 (visit$ or appointment$)).ti,ab
-
(43)
($contin$ adj2 care).ti,ab
-
(44)
or/9–43
-
(45)
8 and 44
Limits
No language limits applied.
Publication Limits: Clinical Trial, Controlled Clinical Trial, Corrected and Republished Article, Evaluation Studies, Journal Article, Meta-Analysis, Multicenter Study, Published Erratum, Randomized Controlled Trial, Retraction of Publication, Review, Review Literature, Technical Report, Validation Studies.
Key
$, truncation; ab, abstract; ti, title; co, complications;/dt, drug therapy;/di, diagnosis;/nu, nursing;/pc, prevention and control;/px, psychology;/th, therapy; and/tm, transmission
Rights and permissions
About this article
Cite this article
Higa, D.H., Crepaz, N., Mullins, M.M. et al. Identifying Best Practices for Increasing Linkage to, Retention, and Re-engagement in HIV Medical Care: Findings from a Systematic Review, 1996–2014. AIDS Behav 20, 951–966 (2016). https://doi.org/10.1007/s10461-015-1204-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-015-1204-x
Keywords
- Linkage to HIV care
- Retention in HIV care
- Engagement in HIV care
- Systematic review
- Evidence-based interventions
- Best practices